Therapeutic effect in a medical use claim under the EPC
Medical use claims under the European Patent Convention (EPC) are considered to include the therapeutic effect as a functional technical feature and are therefore limited to the achievement of such an effect (G 6/88, Headnote and reason 9; G 2/88, Headnote III and reason 9; and, recently, G 2/21, reason 74). This has implications for the evaluation of novelty under Article 54 of the EPC and sufficiency under Article 83 of the EPC of a medical use claim.
On the one hand, for a prior art to be novelty destroying, it must be shown that it discloses the achievement of the therapeutic effect. On the other hand, for a medical use claim to be considered sufficiently disclosed, it must be shown that the application as filed discloses the achievement of the therapeutic effect.
Sufficiency of disclosure under Article 83 of the EPC
Final proof of a therapeutic effect is usually only available at the very end of a drug development process when efficacy is demonstrated by sometimes clinical phase II but usually clinical phase III study results. As acknowledged in the landmark decision T 0609/02, providing such a final proof usually requires many years and very high developmental costs that will only be borne by the industry if protective rights can be obtained. The patent system thus allows a therapeutic effect to be claimed much earlier.
T 0609/02 established the principle of a “plausible” disclosure (providing, for instance, suitable in vitro tests) being enough for demonstrating sufficiency of disclosure of a therapeutic effect under Article 83 of the EPC. However, the board also clarified that a simple verbal statement is not enough (T 0609/02, reason 9). The decision by the Enlarged Board of Appeal (G 2/21) on March 23 2023 confirmed this principle under Article 83 of the EPC, although using the term “credible” disclosure (G 2/21, reason 77).
Novelty under Article 54 of the EPC
As outlined, for example, in T 1437/07, the standard of disclosure for showing achievement of a therapeutic effect established in the context of Article 83 of the EPC (i.e., a “credible” disclosure) is the same standard to be applied in the context of evaluating whether a prior art discloses achievement of a therapeutic effect in the context of novelty (Article 54 of the EPC). Reasons 25 and 26 of T 1437/07 state (emphasis added): “25. A disclosure in a prior art document is novelty-destroying only if the teaching it contains is reproducible. This need for an enabling disclosure is in conformity with the principle expressed in Article 83 EPC. Thus, the requirements of sufficiency of disclosure are identical for a prior art document and a patent.
“26. In accordance with the principles developed by the case law in the framework of the evaluation of the requirements of Article 83 EPC in the case of a medical use, the skilled person should not only be able to carry out the teaching of document R21, but it should also be credible that the effect at issue – here relief of pain – has been achieved.”
The application of the same standard of disclosure under Article 83 of the EPC for patent applications and Article 54 of the EPC for the prior art is, in fact, an old principle already established by the boards of appeal in T 0206/83 of 1986 (reason 2) and T 0576/91 of 1993 (reason 2.1).
The relevant facts of T 0209/22
In T 0209/22, a decision dated March 21 2024, the claim concerned a combination product of umeclidinium and vilanterol for use in the treatment of chronic obstructive pulmonary disease and/or asthma, wherein the product is administered once per day.
For lack of novelty under Article 54 of the EPC, document D21 represents a ClinicalTrials.gov protocol directed to a Phase 1 study assessing the safety, tolerability, and pharmacokinetic and pharmacodynamic effects of GSK573719 and GW642444 (corresponding to the claimed actives) administered individually and concurrently in healthy subjects. The application as filed discloses the same study, but while D21 does not contain the results of the study, the application as filed does. The board did not consider D21 novelty destroying for the claimed subject matter. The inventive step discussion did not consider D21 at all.
Novelty and the relationship with sufficiency of disclosure in T 0209/22
Contrary to previous case law as referred to above, the board in T 0209/22 expressly states in reason 5.6, in the context of a novelty/sufficiency squeeze argument raised by the opponent-appellant, that “different standards indeed apply” to novelty (Article 54 of the EPC) and sufficiency of disclosure (Article 83 of the EPC).
For novelty, the board stated that the study protocol merely relates to healthy subjects, which is not a direct and unambiguous disclosure of a treatment of patients (reason 5.6.1, emphasis added): “To be novelty-destroying, a prior-art disclosure must meet the standard of direct and unambiguous disclosure of the claimed subject-matter. This criterion was not met by D21 and the corresponding clinical study with regard to attaining the claimed therapeutic effect, because the study was performed with healthy subjects (see section 4 above).”
When discussing sufficiency of disclosure under Article 83 of the EPC, the board acknowledged the treatment effect as being rendered credible by, inter alia, the results of the same study performed in healthy subjects. According to the board, for sufficiency, a direct and unambiguous disclosure of a therapeutic effect is not always required. According to reason 5.6.4 (emphasis added): “For the requirement of sufficiency of disclosure to be met, it is not always necessary that attaining the technical effect in question should be disclosed directly and unambiguously in the application as filed (e.g., in the form of results of a clinical phase III trial demonstrating efficacy in the claimed therapeutic use). Rather, as stated in point 5.2 above, the applicable standard is that attaining the technical effect in question has to be credible. This has to be assessed according to the circumstances of each individual case.”
Discussion
According to the board in T 0209/22, different standards apply in the context of Article 83 of the EPC and Article 54 of the EPC. While a prior art must meet the standard of direct and unambiguous disclosure to be novelty destroying, a direct and unambiguous disclosure of the therapeutic effect in the application as filed is not always required for sufficiency, according to the board. Under Article 83 of the EPC, the applicable standard is a credible disclosure, as depicted below.
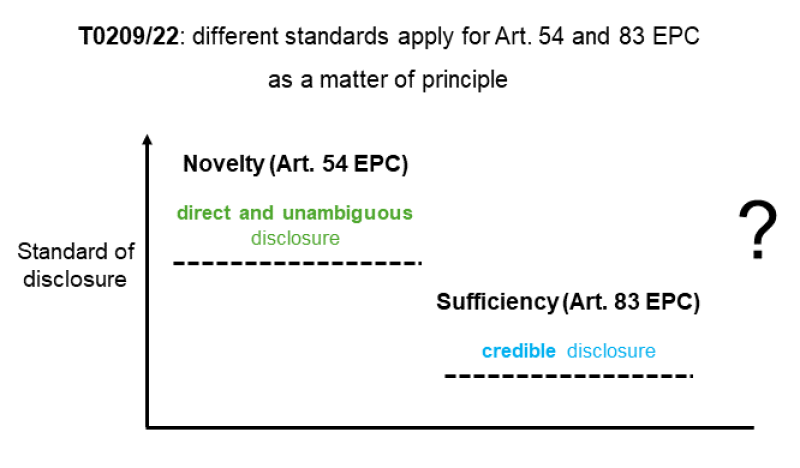
The findings of the board in T 0209/22 seem to create a tension with long-standing case law, such as decision T 1437/07, holding that the requirements of sufficiency of disclosure are identical for a prior art document and a patent/application.
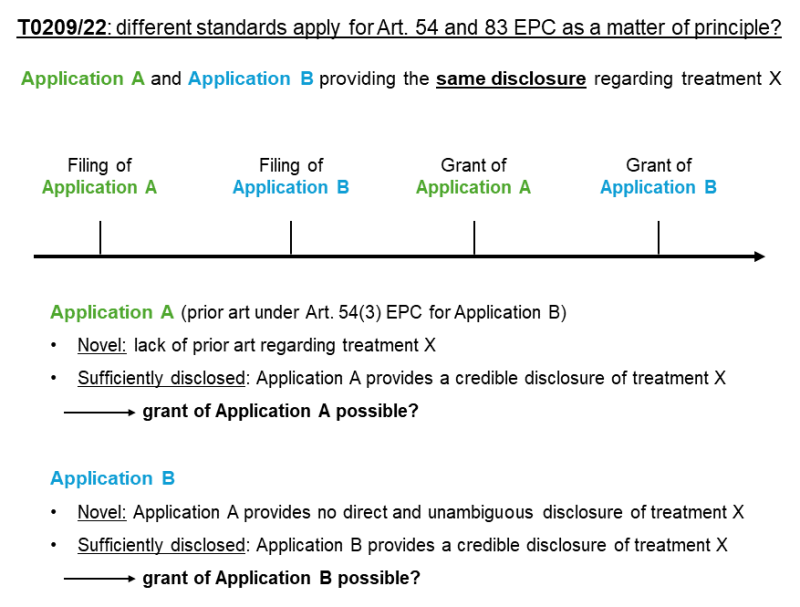
The approach taken by the board in T 0209/22 may raise questions concerning the consistency of the patentability requirements under the EPC. Should the standard be different as a matter of principle, the very same disclosure may be enough to establish sufficiency of disclosure, while it would not be enough to establish novelty-destroying disclosure. As a consequence, the same subject matter could be claimed several times.
In the event of two identical but consecutively filed applications, where the earlier application is prior art under Article 54(3) of the EPC relevant for novelty only, the earlier application would support a grant of a patent but would not prohibit the granting of a second patent for the same subject matter based on the later application because of the higher standard under Article 54 of the EPC, as illustrated below.
While T 0209/22 seems the first decision to expressly state that different standards apply under articles 54 and 83 of the EPC, other decisions rendered in the context of clinical trial protocol disclosures also seem to apply a higher standard concerning novelty than the “credible disclosure” standard under Article 83 of the EPC. The board in T 0239/16, for example, required, in the context of novelty under Article 54 EPC, a therapeutic effect to “arise with certainty” from the prior art (see reason 5.2).
It remains to be seen how the case law on the standard of disclosure with respect to novelty will develop. The new referral G 1/23 also concerns the standard of disclosure for novelty, albeit in a different context; namely, the reproducibility of certain aspects of a product available in the prior art.