On November 22 2022, the CNIPA made invalidation decision No. 58648 and declared Markush claim 1 of AbbVie’s patent ZL201510165051.4 titled ‘apoptosis-inducing agents for the treatment of cancer and immune and autoimmune diseases’ (the Patent) invalid, citing lack of novelty.
The Patent relates to the blockbuster drug venetoclax, which is the first oral and selective B-cell lymphoma factor-2 (Bcl-2) inhibitor, jointly developed by AbbVie and Roche, to treat chronic lymphocytic leukemia and acute myelocytic leukemia. In 2021, AbbVie generated a sales revenue of $1.82 billion from venetoclax, which put it in fifth place among all the biopharmaceutical’s marketed drugs.
Background to the claim
Venetoclax was first launched in the US market in April 2016. On December 8 2020, AbbVie announced that it had secured conditional approval from the National Medical Products Administration (NMPA) to launch venetoclax in China. Venetoclax, which was marketed as Venclexta, was the first, and remains the only, NMPA-approved Bcl-2 inhibitor in China.
Three patents, including the patent at issue, are registered on China’s patent information platform of listed drugs as being pertinent to Venclexta, which falls under the protection scope of Markush claim 1 of the Patent, among others. The invalidation decision undermines the stability of the Venclexta Chinese patent portfolio and delivers a blow to AbbVie.
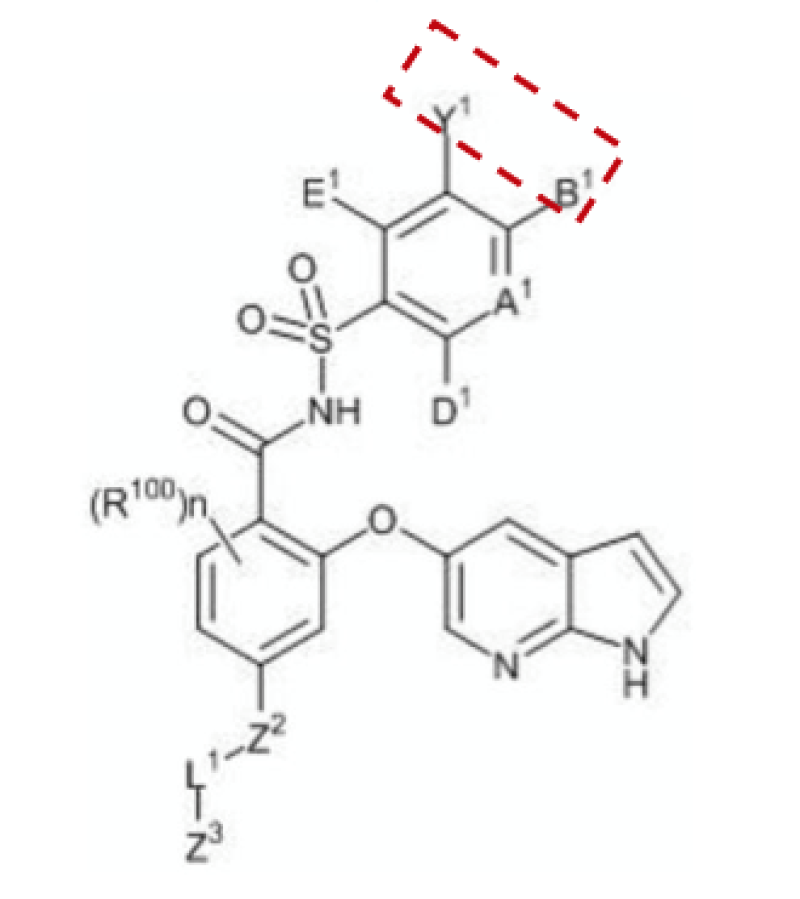
Markush claim 1 (as shown below) reads: “Wherein the cyclic moiety represented by Y1 and B1 together… is unsubstituted or independently substituted by 1-5 substituents below… R57A is spiroalkyl or heterospiroalkyl…”
The priority document records the Markush formula and its only difference from claim 1 is that R57A is a spiroalkyl.
The CNIPA’s ruling
The CNIPA found that claim 1 cannot enjoy priority, so evidence 1 submitted by the petitioner is prior art. It thus concluded that claim 1 is devoid of novelty with respect to evidence 1.
In the invalidation procedure, AbbVie asserted that R57A in claim 1 only includes spiroalkyl and heterospiroalkyl, which can be divided into two parallel technical solutions, and the deletion of R57A as a heterospiroalkyl group should be allowed.
To back up its argument, AbbVie submitted invalidation decision No. 24591 to prove that there has been precedent where the deletion of substituents in Markush claims is allowed in the invalidation procedure. AbbVie contended that after deleting the heterospiroalkyl group, amended claim 1 is consistent with the priority document and can enjoy priority. Under such circumstances, evidence 1 does not constitute prior art and has no bearing on the novelty assessment of claim 1.
The CNIPA rejected AbbVie’s argument based on the following reasoning: although the definition of R57A in claim 1 only includes spiroalkyl and heterospiroalkyl groups, there are still dozens of other substituents, as in nature a Markush claim is an overall technical solution, rather than an assembly of different compounds. Furthermore, the description fails to convey that spiroalkyl and heterospiroalkyl groups are studied as different inventive concepts.
The CNIPA therefore rebutted AbbVie’s argument that claim 1 can be divided into two parallel technical solutions, based on the definition of R57A. The deletion of R57A as a heterospiroalkyl group is therefore not the deletion of a technical solution and shall not be allowed. In addition, the description of the Patent introduces six embodiments of R57A as heterospiryl groups, which are not included in the priority document. Therefore, Markush claim 1 with R57A as a heterospiryl group should not enjoy priority, otherwise it will harm the public interest.
The CNIPA dismissed invalidation decision No. 24591 submitted by AbbVie, finding it irrelevant to this case.
Implications of the decision
The decision reaffirms that for Markush claims, CNIPA examination practice still follows the principle set by the Supreme People's Court in its decision Zui Gao Fa Xing Zai No. 41 (2016):
A Markush claim should be deemed as a collection of Markush elements, rather than a collection of many compounds, and Markush elements can only be expressed as a single compound under certain circumstances; and
In an invalidation procedure, the amendment of Markush claims must be strictly restricted. Allowing the deletion of any option of a variable group will deprive the public of a stable expectation and is detrimental to the stability of the patent regime.
Invalidation decision No. 24591 adduced by AbbVie may shed some light on the exceptional circumstances under which Markush elements can be expressed as a single compound. In this decision, the patentee amended the Markush claim into a specific compound by deleting the definition of related substituents. The amendment was allowed by the CNIPA as the said compound is the only compound prepared in the description and the core of the invention, and its active effects have been tested.
The CNIPA believes that the acceptance of the above amendment fully reflects the legislative intent of the Patent Law in encouraging innovation and is conducive to focusing on the technical contributions in assessing inventiveness.
This case may serve as a point of reference in terms of drafting compound patents incorporating Markush claims. Where priority is claimed, the patentee needs to ensure that the Markush claims are consistent with the previous application to the largest extent possible. In order to provide support for possible amendments, patentees are also strongly advised to build a multi-level claim system during the drafting process and to fully disclose core invention if possible.