After the product patent had expired, the brand name pharmaceutical company sued generic drug manufacturers based on a manufacturing process patent. The Tokyo District Court affirmed infringement under the doctrine of equivalents (DOE) for the medicinal compound's manufacturing process patent.
Summary of the case
The plaintiff, Chugai Pharmaceutical, jointly owns a patent for a manufacturing process of compounds including maxacalcitol. Chugai manufactures and sells Oxarol ointment, a therapeutic agent of keratosis, whose active ingredient is maxacalcitol.
Chugai had owned a product patent regarding maxacalcitol. This patent expired on December 26 2010 after the patent term was extended.
The defendants Iwaki Seiyaku etc sold generic Oxarol ointments and the defendant DKSH Japan had imported the active ingredients of the generic product from Cerbios Pharma and sold it.
Chugai sought an injunction against the defendants' importation and assignment claiming that the defendants' manufacturing process of maxacalcitol preparations and their API was equivalent to the patented process.
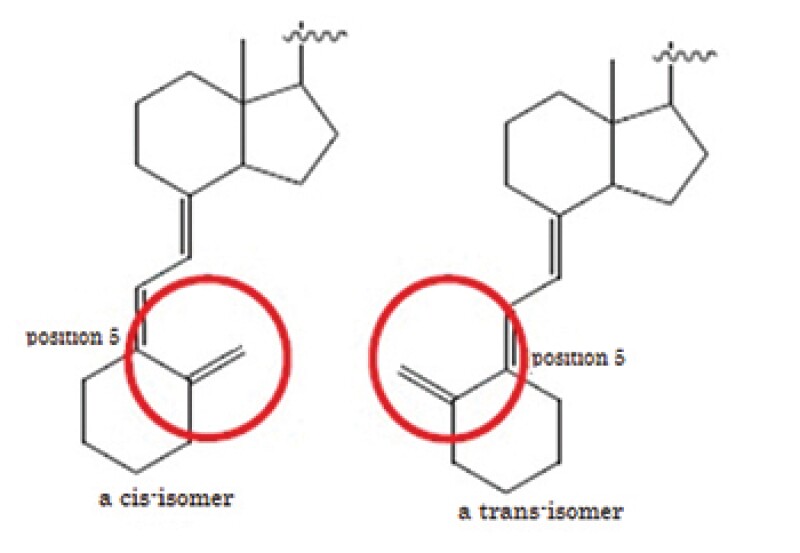
The defendants' process does not literally satisfy the claim elements because the structure of a starting material and an intermediate of the patented process is cis-isomer whereas that of defendants' process is trans-isomer. The main issue was DOE.
Judgment of December 24 2014, Tokyo District Court
The Tokyo District Court (Presiding Judge Shimasue) affirmed satisfaction of five requirements of the ball spline case (judgment of February 24 1998, Supreme Court) and held that the defendants' process is equivalent to the Chugai invention. In February 2015, the Court granted a preliminary injunction.
(1) First requirement of DOE
The "essential element of the patented invention" refers to a characteristic part among the structures stated in the scope of claims in the description. The characteristic part should constitute the core of the technical idea that establishes the means to solve the problem specific to the patented invention.
The invention obtains an effect that can reduce the number of manufacturing processes of maxacalcitol compared with the prior art. To obtain this effect, the invention adopts using a two-step reaction and introducing a side chain of maxacalcitol which is the important part that establishes the means to solve the problem.
The defendants' process shares the important part of the means to solve the problem with the invention using the two-step reaction. It is not significant in the means to solve the problem whether a starting material and an intermediate is a cis-isomer or a trans-isomer.
Thus, in the case where the objective material has vitamin D structure, whether a starting material and an intermediate is a cis-isomer or a trans-isomer is not the "essential element of the patented invention".
Therefore, the defendants' process satisfies the first requirement of DOE.
(2) Second requirement of DOE
The defendants' process can achieve the purpose of the invention which can reduce a number of manufacturing processes of maxacalcitol by replacing a cis-isomer with a trans-isomer in a starting material and an intermediate and the same effect as the invention can be obtained because the defendants' process uses the two-step reaction.
Therefore, the defendants' process satisfies the second requirement of DOE.
(3) Third requirement of DOE
The process to manufacture a cis-isomer vitamin D derivative by using a trans-isomer compound as a starting material and introducing a side chain properly was already known to a person skilled in the art at the time of the priority date.
Thus, a person skilled in the art could easily come up with the idea of the defendants' process which replaces a cis-isomer in a starting material of vitamin D structure with a trans-isomer and which finally converts a trans-isomer into a cis-isomer at the time of the practicing of the defendants' process.
Therefore, the defendants' process satisfies the third requirement of DOE.
(4) Fourth requirement of DOE
The defendants allege that the defendants' process could have been easily conceived by a person skilled in the art on the basis of the technology in the public domain such as an invention in D4 at the time of the priority date.
There are two differences between the defendants' process and D4 invention. One is that D4 invention does not disclose a part of the defendants' process (difference 1). Another is that an objective material of D4 invention is not maxacalcitol (difference 2).
Regarding difference 2, it is reasonable to find that a person skilled in the art could have easily conceived to use a starting material of D4 invention as a starting material and to set maxacalcitol as an objective material instead of an objective material of D4 invention. However, regarding difference 1, part of the defendants' process was not disclosed or suggested at all in D4. Thus, difference 1 could not have been easily conceived by a person skilled in the art.
Therefore, the defendants' process satisfies the fourth requirement of DOE.
(5) Fifth requirement of DOE
In the description, the words which clearly express the distinction between a cis-isomer and a trans-isomer are not used. Further, the circumstance where the patent was registered because of the differences from prior art which uses a trans-isomer is not found.
Thus, in the case where a starting material or an intermediate has a vitamin D structure, it is not found that the invention had been intentionally limited to a cis-isomer or a trans-isomer had been intentionally excluded.
Therefore, the defendants' process satisfies the fifth requirement of DOE.
Practical tips
This judgment attracts attention as it affirmed infringement under DOE for a medicinal compound's manufacturing process patent although manufacturing process patent infringement is rarely found and only few judgments granted DOE after the ball spline Supreme Court judgment. This case could be evaluated that LCM strategy using manufacturing process patent after the product patent had expired went successful.
The IP High Court designated the appeal case as en banc and the judgment is due on March 25 2016. The IP High Court judgment should be watched closely.

|

|
Takanori Abe |
Michiko Kinoshita |
ABE & PartnersMatsushita IMP Building1-3-7, Shiromi, Chuo-ku, Osaka, 540-0001, JapanTel: +81 6 6949 1496Fax: +81 6 6949 1487abe@abe-law.comwww.abe-law.com










